- Apr 15, 2025
Are Prokinetics Effective in Rabbits? My Thoughts on the Latest JAVMA Study
- Kristen Turner
- Journal club
- 0 comments
A new article just dropped in JAVMA noting no measurable effect of 4 gastrointestinal motility drugs in healthy rabbits. With increasing debate on how we should and shouldn’t treat rabbit gastrointestinal syndrome (RGIS), I wanted to share my current protocol and the evidence behind it.

What Is Rabbit Gastrointestinal Syndrome (RGIS)?
First of all, we need to define RGIS. Other common names are ileus, gastric hypomotility or simply, GI stasis. This can be a primary gastrointestinal issue, however, we are increasingly recognizing that RGIS, particularly when recurrent, is almost always a secondary problem.
During RGIS, the affected rabbit has some sort of issue that causes pain or stress, leading to anorexia. Decreased intake of fiber leads to reduced motility throughout the gastrointestinal tract and ultimately rapid die-off of cecal bacteria. As normal flora die off, opportunistic pathogens, such as Eschericia coli, Pseudomonas aeruginosa and Clostridium spp. proliferate, leading to dysbiosis, and if left untreated, ultimately can lead to death.
Pain or stress → anorexia → reduced fiber intake → slowed gut motility → cecal dysbiosis → potential death
Common Underlying Conditions Leading to RGIS
Dental disease
Liver lobe torsion
Urolithiasis
Encephalitozoonosis (E. cuniculi infection)
Inappropriate diet
-
Anything that causes pain or stress
Clinical Signs and Diagnostic Clues in Suspected RGIS
Presenting complaint:
Anorexia
Reduced or absent fecal production
‘Diarrhea’ (usually uneaten cecotrophs or cecal dysfunction)
Lethargy
Dark urine
On Physical Exam:
Empty or doughy stomach
OR Gas/fluid-distended stomach
Dehydration
Diarrhea or soiled hind end
-
Hypothermia (rectal temperature <100.4℉ or <38℃)
Poor prognostic indicator
3x higher risk of death than normothermic rabbits (Di Girolamo et al. 2016)
Key point: RGIS is usually a sign of a deeper issue. Don’t just treat and turf.
Recommended Diagnostic Workup
In an ideal scenario, I perform a thorough physical exam, administer an analgesic +/- mild sedative (opioid of choice +/- midazolam) and provide thermal support, and then draw for a full complete blood count and biochemistry and take full body radiographs.
If radiographs do not give a definitive cause, I will perform abdominal ultrasound, skull radiographs, and/or CT, particularly if I’m suspicious of a dental issue or liver lobe torsion. If E. cuniculi status is unknown I will also draw blood for titers.
When providing contextualized care, my minimums are a thorough physical exam, analgesic/sedative administration and a packed cell volume and blood glucose. If an iStat or other point-of-care machine is available, those cartridges often provide the minimum analytes needed at a reduced price (PCV, BG, BUN, Crea) relative to full complete blood count and chemistry analyzers.
If initial treatment is not rewarding, it is vital to run (if not originally done) or recheck a minimum database as underlying disease such as pyelonephritis, liver lobe torsion, or obstruction may be present and the signs of RGIS will not resolve until the primary issue is treated.
Common Lab Findings in RGIS
Often, laboratory findings are nonspecific with RGIS, especially if it is occurring secondary to a chronic process like dental disease.
Hemoconcentration
Mild heterophilic leukocytosis +/- lymphopenia (stress leukogram)
Hyperglycemia (note levels >450 mg/dL or 25 mmol/L raise concern for obstruction)
-
Azotemia
Often pronounced
Since USG is not a good indicator of renal function in rabbits, recheck after 24 hours of fluid therapy to determine pre-renal vs. true kidney injury
-
Hypercalcemia (remember this can be normal in rabbits due to passive absorption of calcium and is not typically a pathologic finding)
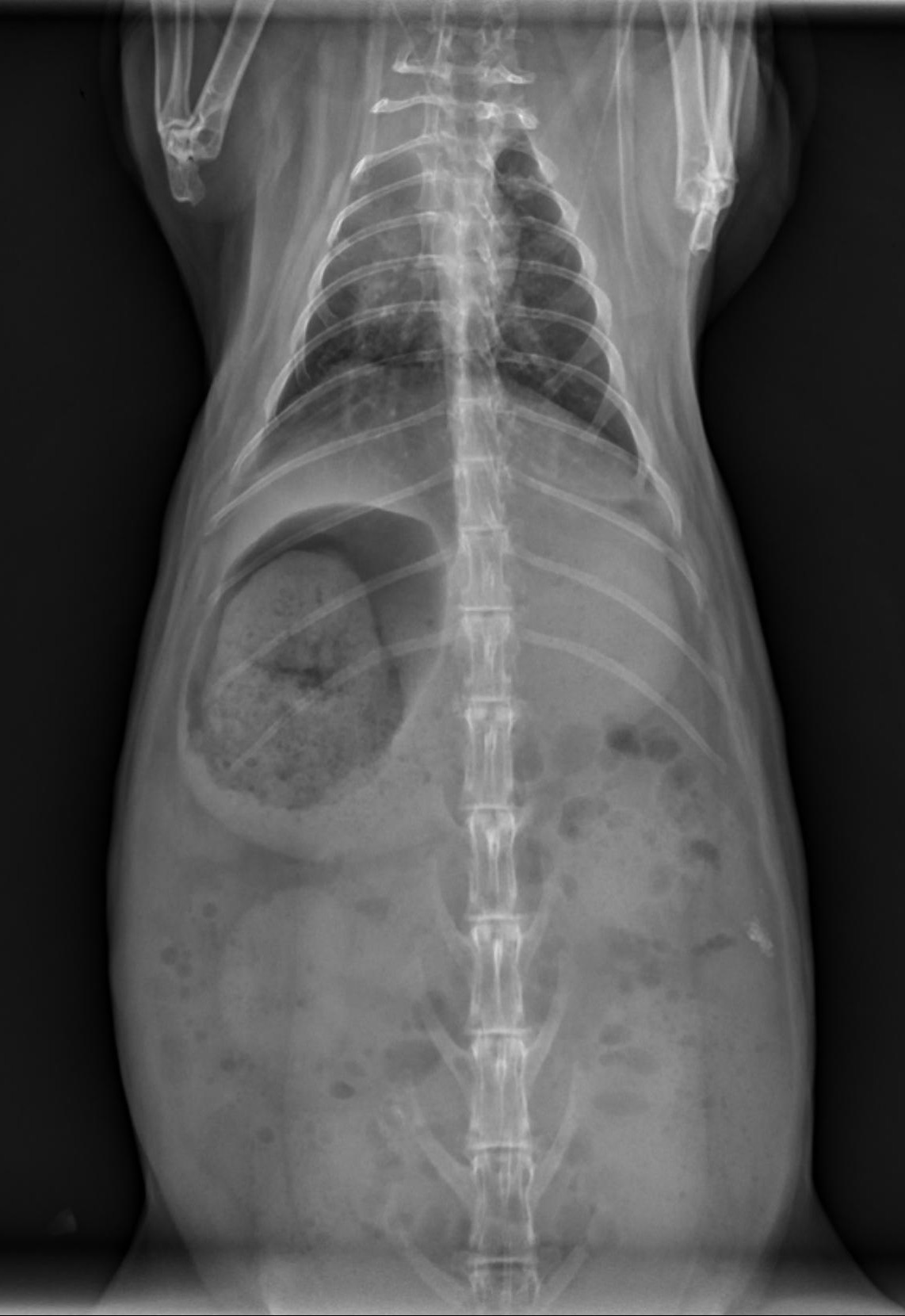
Radiographic Patterns: Ileus vs. Gastric Outflow Obstruction
Radiographs can help differentiate between RGIS and a potentially more serious gastric outflow obstruction (although to be honest, I approach treatment the same way for both in the first 24 hours).
RGIS radiographic signs:
Dehydrated, radiodense stomach contents
Circumferential line of gas around dehydrated contents
+/- cecal distension
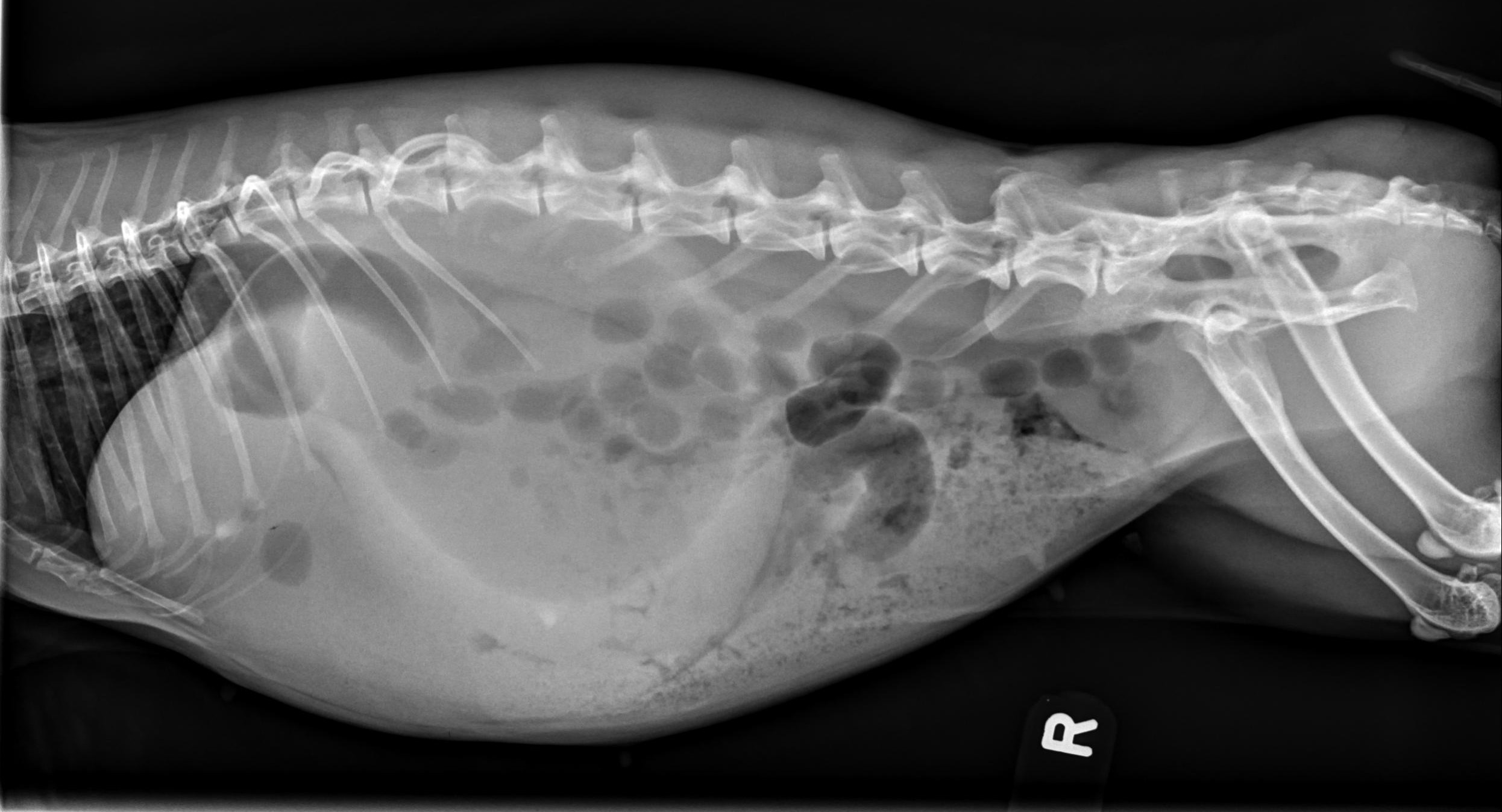
Gastric outflow obstruction:
Enlarged, fluid-filled stomach
Dorsal gas cap
Stomach extends beyond last rib
2 populations of small intestine often present
Keep an Eye Out for:
Mild anemia ➡️ consistent with dental or other long-term disease (anemia of chronic disease)
Moderate to severe anemia + elevated ALT ➡️ concerning for a liver lobe torsion
Look carefully for urolithiasis ➡️ sometimes uroliths hide out in typical rabbit bladder sludge
Stabilization: Rehydration and Analgesia Are Key
Hospitalization is strongly recommended to initiate:
Thermal support with warmed IV fluids, incubators, forced warm-air blankets, etc
-
IV crystalloids (calculate specifically for your patient - don’t just use 2-3x maintenance)
100+ mL/kg/day maintenance
Fluid deficit (calculate using % dehydration)
Resuscitate over 12-24 hours
If unstable, start with ¼ shock bolus (90 mL/kg) over 15 minutes
-
Ideal scenario - monitor blood pressure and titrate fluids to effect
Not typically practical unless in a very large rabbit or specialty facility with advanced equipment/staffing
-
Colloids such as hypertonic saline and hetastarch can also be used for volume resuscitation, but are not typically part of my protocol.
Keep in mind, a 2010 meta-analysis in humans suggested that synthetic colloids may increase the risk of AKI and death - however, this link has not been established in veterinary medicine (Wiedermann et al. 2010).
Lidocaine CRI for GI Stasis: A Game Changer?
A recent paper out of the University of Wisconsin-Madison demonstrated that rabbits presenting for gastrointestinal obstructions had an increased survival rate (nearly 8x!) when administered a lidocaine continuous rate infusion (Huckins et al. 2023).
A previous paper demonstrated that the sodium-channel blocker provided better analgesia when administered to rabbits post-ovariohysterectomy as compared to buprenorphine, demonstrated by increased GI motility, increased fecal pellet production, reduced heart rate and more ‘normal’ behaviors (Schnellbacher et al. 2017).
Based on these 2 papers, a lidocaine CRI has become my treatment of choice for gastrointestinal stasis, in combination with opioid analgesia.
Slow (over ~5 minutes) IV loading dose of 2 mg/kg
Continue at 50-100 mcg/kg/min
Online CRI calculators can help you with the math. You can give the CRI separately via a syringe pump or can give as part of the IV fluids
Lidocaine is light-sensitive so be sure to wrap or cover the bag
-
Don’t forget to label your fluid bag with what and how much you added!

My Take on Opioid Analgesia in RGIS
There is some debate on the effect of opioids on gastrointestinal motility, and several studies have proven that multiple opioids do adversely affect rabbit gastrointestinal motility (Defflers et al. 2018, Defflers et al. 2022, Feldman et al. 2021, Schnellbacher et al. 2017).
However, those effects seem to be transient, with healthy rabbits regaining normal motility without intervention. We also have to weigh the fact that pain is always going to adversely affect GI motility so we have to determine which is the greater evil.
In my experience, most rabbits recover with opioid analgesia, and since it is the most proven analgesia we have, I do choose to administer them.
There is even further debate on the opioid of choice, but ultimately it often comes down to what you have available.
While there is some anecdotal and literature evidence that buprenorphine slows motility more than other opioids, at many practices it is the only opioid I have available and I still have rabbits fully recover within 24-48 hours.
Many veterinarians use methadone, however a recent study showed relatively poor bioavailability of methadone in rabbits when administered IM or SQ (Pujol et al. 2023).
When and How to Start Assisted Feeding
Once obstruction is ruled out (or after orogastric decompression of the stomach + several hours of fluids/analgesics), I begin assisted feeding every 4-6 hours using a critical care herbivore formula until the rabbit is eating well on its own.
Do Prokinetics Help Rabbits with RGIS?
The short answer: Not really - and here’s why…
While prokinetics like metoclopramide and cisapride are widely used, evidence in rabbits is limited and weak:
-
Mostly ex vivo studies from the 1970s-90s (Okwuasaba and Hamilton 1976, Langer and Bramlett 1997)
Ex vivo means the intestines were harvested from rabbits and the drugs were tested directly on the tissue
These studies did show improved contractility with both metoclopramide & cisapride
-
Sparse PK/PD data
The PK study on metoclopramide showed good but transient bioavailability at 2 mg/kg (4x the formulary dosage) when injected intraarterial (IA), IM, or SQ (De Vito et al. 2015)
The only PK study on cisapride was performed at 5-10x the formulary dosage (Michiaels et al. 1987)
Retrospective studies typically use combinations of drugs, making them hard to interpret (Schuhmann and Cope 2014)
-
Very limited safety and efficacy studies to guide dose formulation
Cisapride at 0.5 mg/kg q8hrs for 2 days showed no effect on GI transit time, fecal output or food/water consumption alone or combined with buprenorphine (Feldman et al. 2021)
Most dosage recommendations in formularies refer back to other formularies or textbooks, not actual prospective studies
However, none of the mentioned studies showed significant adverse effects at formulary dosages
Believe it or not, this is ALL of the literature that exists in regard to rabbits and the traditional prokinetics that many of us have recommended for years: the rest of our evidence is anecdotal.
What About Appetite Stimulants in Rabbits?
The usage of appetite stimulants in the treatment of RGIS has grown, so I want to briefly mention these.
Capromorelin:
Stimulates food intake and improve gastric and intestinal motility in research models and in humans
Increased overall feed intake and fecal output in healthy rabbits compared to control rabbits when dosed at either 4 mg/kg or 8 mg/kg PO twice daily (Draper et al. 2022).
In rabbits, capromorelin was found to be less effective at stimulating appetite than mirtazapine in healthy rabbits. However, neither drug was found to be particularly effective at increasing appetite in post-castration rabbits when dosed once daily (Draper et al. 2022).
Mirtazapine:
-
More effective than cisapride at stimulating appetite and fecal output at 0.5 mg/kg and 1 mg/kg transdermal once daily in healthy rabbits
However, still not effective in post-surgical rabbits (Draper et al. 2022)
-
Oral administration at 3 mg/kg once daily led to increased fecal output, but no effect on food intake in healthy rabbits
These rabbits had mild weight loss the following week, therefore close monitoring is indicated if utilized (Ozawa et al. 2022)
Both appetite stimulants did seem to be safe with pinnal erythema in the rabbits administered transdermal mirtazapine the only observed adverse effect in the above studies.
Maropitant: GI prokinetic, visceral analgesic, or just hype?
Finally, I want to touch on maropitant, as it is getting a lot of anecdotal usage for all sorts of things, including RGIS.
-
A PK study from 2022 indicates rabbits have plasma concentrations similar to dogs 24 hours post-administration with 1 mg/kg maropitant citrate IV or SQ
Incidentally, researchers noted rabbits receiving maropitant had increased fecal production (Ozawa et al. 2019)
-
A safety and efficacy study evaluating maropitant administered to post-surgical rabbits at 2 mg/kg or 10 mg/kg once along with multi-modal analgesia didn’t find statistically significant differences vs controls, indicating no effect on GI motility
The high dose group showed a decrease in food intake and fecal output, so high dosages may actually inhibit motility (Roeder et al. 2023)
Otherwise, no adverse effects were noted
-
A subsequent study was similarly structured utilizing maropitant at 2 or 4 mg/kg SQ once in post-surgical rabbits and focused on pain scores, in addition to food intake and fecal output
Food intake and fecal production were difficult to interpret due to the multi-institutional nature of the study
Rabbits that received the 4 mg/kg dose had significantly lower pain-related behavior scores post-operatively, indicating potential visceral analgesia (Grayck et al. 2024)
Ultimately, I remain skeptical on maropitant’s potential prokinetic ability, however, anecdotal usage and Grayck et al.’s study (2024) suggest potential for use as a visceral analgesic. We also know that maropitant is utilized as an antiemetic in other species, and while rabbits cannot vomit, it’s entirely possible they may experience nausea, so maropitant may be effective at improving overall patient comfort and encouraging food intake.
Finally - Let’s Talk About the New JAVMA Study
A new paper out of Oklahoma State University College of Veterinary Medicine evaluated the effect of 4 prokinetics in healthy New Zealand White rabbits.
The study found that metoclopramide, cisapride, pyridostigmine, and capromorelin had no effect on food intake, water intake or fecal and urine production when administered once at the recommended dosages in Carpenter’s Exotic Animal Formulary (Di Girolamo et al. 2025).
Some limitations of this study:
-
Only a single oral dose of each drug was administered
Notably - the metoclopramide pharmacokinetic study was at a higher dose (2 mg/kg vs. 0.5 mg/kg) and that study did not evaluate the oral route.
Clinically, it’s rare we would give a single dose of one of these medications
The study was performed in healthy rabbits, not those with motility issues
Compounded medications were utilized
Small sample (10 rabbits) of a single breed
Maropitant and mirtazapine were not evaluated
Just because the study had limitations, doesn’t mean we should ignore it. It’s a well-structured prospective study that shows us what we have suspected for a while - most likely, prokinetics are not working as well as we had hoped at improving motility in rabbits.
However, the authors of the paper specifically point out that this is a starting point, not meant to be the final word on prokinetics.
We Still Need More Research
Structuring an RGIS study that controls for all possible variables is going to be extremely difficult.
Some potential variables that would needed to be controlled are:
-
Inclusion criteria for the study
Duration/type of clinical signs?
Do we include rabbits with obvious primary issues like dental disease?
What about E. cuniculi status?
Hospitalized vs. outpatient care
Are the patients receiving otherwise standardized treatment (similar IV fluid protocols, analgesic administration, etc)?
Do we include rabbits with suspected gastric outflow obstruction?
If this is a multi-institutional study, how are we standardizing these things?
Who is evaluating pain in the patients and what system is being utilized?
And we need these studies for all of the drugs discussed above. It’s not an easy undertaking.
So Are Prokinetics Worth Using in Rabbits with RGIS?
While I don’t regularly utilize prokinetics while treating RGIS, drawing the conclusion that prokinetics should never be utilized in rabbits with RGIS is short-sighted.
We need further data to be able to conclude prokinetics have no place in RGIS.
While we don’t have obvious evidence they work, there is also no evidence they are harmful in the absence of obvious gastric outflow obstruction (besides to the client’s wallet).
I don’t advocate utilizing prokinetics over proven treatments, but if clients are insistent, or if the patient is not responding to initial treatment and facing euthanasia, it may be worth a try as long as there is no obvious obstruction.
Practicing Evidence-Based Medicine Means Staying Flexible
One of the hardest things about being an exotics vet today? The field is constantly evolving.
We must stay adaptable and willing to move away from what’s “always worked” in favor of what the evidence supports.
Let me know your thoughts - do you still use prokinetics? What has your experience been like with RGIS treatment?
References:
1. De Vito V, Kim T-W, Rota S, et al. Pharmacokinetics of Metoclopramide After IntraARTERIAL, Intramuscular, Subcutaneous, and Perrectal Administration in Rabbits. Journal of Exotic Pet Medicine 2015;24:361–366.
2. Deflers H, Gandar F, Bolen G, et al. Influence of a single dose of buprenorphine on rabbit (Oryctolagus cuniculus) gastrointestinal motility. Veterinary Anaesthesia and Analgesia 2018;45:510–519.
3. Deflers H, Gandar F, Bolen G, et al. Effects of a single opioid dose on gastrointestinal motility in rabbits (oryctolagus cuniculus): comparisons among morphine, butorphanol, and tramadol. Veterinary Sciences 2022;9:28.
4. Di Girolamo N, Maranville RE, Pathak D, et al. The 4 prokinetic drugs metoclopramide, cisapride, pyridostigmine, and capromorelin do not increase fecal output or food intake in healthy New Zealand rabbits (Oryctolagus cuniculus). javma 2025:1–7.
5. Di Girolamo N, Toth G, Selleri P. Prognostic value of rectal temperature at hospital admission in client-owned rabbits. javma 2016;248:288–297.
6. Draper JM, Savson DJ, Lavin ES, et al. Comparison of Effects of Capromorelin and Mirtazapine on Appetite in New Zealand White Rabbits ( Oryctolagus cuniculus). j am assoc lab anim sci 2022;61:495–505.
7. Feldman ER, Singh B, Mishkin NG, et al. Effects of cisapride, buprenorphine, and their combination on gastrointestinal transit in New Zealand White rabbits. J Am Assoc Lab Anim Sci 2021;60:221–228.
8. Fisher P, Graham J. Rabbits. In: Carpenter J, Harms C, eds. Carpenter’s Exotic Animal Formulary. 6th ed. St. Louis: Elsevier, 2023;575–625.
9. Grayck M, Sullivan MN, Boscan P, et al. Use of subcutaneous maropitant at two dosages for pain management in domestic rabbits (Oryctolagus cuniculus) undergoing elective ovariohysterectomy or orchiectomy. Topics in Companion Animal Medicine 2024;61:100888.
10. Huckins GL, Tournade C, Patson C, et al. Lidocaine constant rate infusion improves the probability of survival in rabbits with gastrointestinal obstructions: 64 cases (2012–2021). J Am Vet Med Assoc 2024;262:61–67.
11. Langer JC, Bramlett G. Effect of prokinetic agents on ileal contractility in a rabbit model of gastroschisis. Journal of Pediatric Surgery 1997;32:605–608.
12. Michiels M, Monbaliu J, Hendriks R, et al. Pharmacokinetics and tissue distribution of the new gastrokinetic agent cisapride in rat, rabbit and dog. Arzneimittelforschung 1987;37:1159–1167.
13. Okwuasaba FK, Hamilton JT. The effect of metoclopramide on intestinal muscle responses and the peristaltic reflex in vitro. Can J Physiol Pharmacol 1976;54:393–404.
14. Ozawa SM, Hawkins MG, Drazenovich TL, et al. Pharmacokinetics of maropitant citrate in New Zealand White rabbits (Oryctolagus cuniculus). ajvr 2019;80:963–968.
15. Ozawa S, Thomson A, Petritz O. Safety and efficacy of oral mirtazapine in New Zealand White rabbits (Oryctolagus cuniculus). Journal of Exotic Pet Medicine 2022;40:16–20.
16. Pujol J, Vergneau-Grosset C, Beaudry F, et al. Pharmacokinetics and innocuity of a single dose of intravenous, intramuscular, and subcutaneous methadone in the domestic rabbit (Oryctolagus cuniculus). Journal of Exotic Pet Medicine 2023;47:41–46.
17. Roeder M, Boscan P, Rao S, et al. Use of maropitant for pain management in domestic rabbits (Oryctolagus cuniculus) undergoing elective orchiectomy or ovariohysterectomy. Journal of Exotic Pet Medicine 2023;47:14–20.
18. Schnellbacher RW, Divers SJ, Comolli JR, et al. Effects of intravenous administration of lidocaine and buprenorphine on gastrointestinal tract motility and signs of pain in New Zealand White rabbits after ovariohysterectomy. ajvr 2017;78:1359–1371.
19. Schuhmann B, Cope I. Medical treatment of 145 cases of gastric dilatation in rabbits. Veterinary Record 2014;175:484–484.
20. Wiedermann CJ, Dunzendorfer S, Gaioni LU, et al. Hyperoncotic colloids and acute kidney injury: a meta-analysis of randomized trials. Crit Care 2010;14:R191.
Stay in the loop on exotic veterinary medicine
Join my email list to get weekly newsletters to keep you in-the-know in exotic vet med and be the first to receive access to new resources, courses, masterclasses, and more!